
by MM360 Staff | Oct 29, 2020 | Featured News, Myeloma News
Administering radiation therapy to multiple myeloma patients prior to the administration of CAR T cells was found to be safe and undisruptive to CAR T therapy, according to a new study from researchers in the Abramson Cancer Center at the University of Pennsylvania....
by MM360 Staff | Oct 14, 2020 | Featured News, Myeloma News
Cytovia Therapeutics has entered a research and licensing agreement with Inserm, the French National Institute of Health and Medical Research, to develop NK engager bi-specific antibodies and iPSC CAR NK cell therapy targeting CD38, a key marker of multiple myeloma....
by MM360 Staff | Oct 7, 2020 | Featured News, Myeloma News
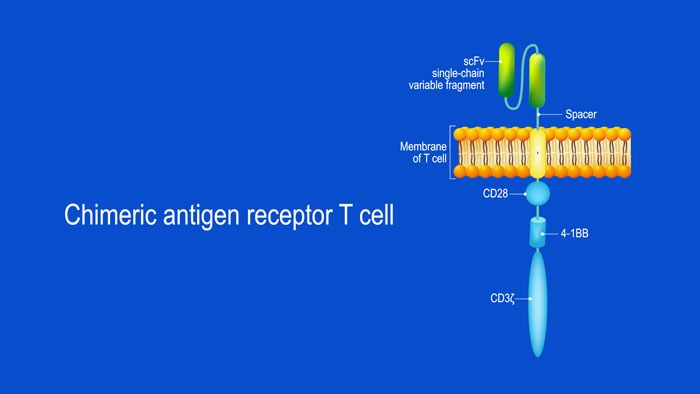
Chimeric antigen receptor (CAR) T cell therapy has shown remarkable successes in fighting B cell leukemias/lymphomas and numerous CAR-T’s are being developed for use in multiple myeloma. While primising results have been reported with BCMA CAR T cells, responses...

by MM360 Staff | Oct 6, 2020 | Featured News, Myeloma News
The success of T cell–mediated therapeutics depends, in part, on the selective expression of cell surface target epitopes to minimize the off-tissue toxicity. B-Cell Maturation Antigen (BCMA) is a protein that is selectively expressed on late-stage B cells and plasma...

by MM360 Staff Writer | Oct 1, 2020 | Featured News, Myeloma News
House Oversight Committee hold hearings on drug Pricing. The Committee is holding two days of hearings with top executives of major drug companies to examine their pricing practices for some of the costliest drugs in the United States. The hearings, titled...

by MM360 Staff | Oct 1, 2020 | Featured News, Myeloma News
Oncopeptides announced today that the Melflufen open-label Expanded Access Program, sEAPort, for eligible U.S. patients, is formally open. EAPs are designed to provide patients living with serious or life-threatening conditions access to investigational medicines when...







