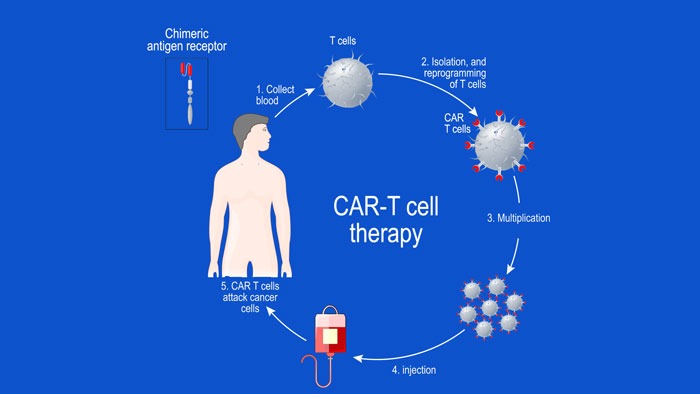
In this article, we look at some of the early-phase clinical trials, where chimeric antigen receptor (CAR) T-cell therapy has been targeted to the B-cell maturation antigen (BCMA) in patients with relapsed or refractory MM (RRMM). The anti-BCMA CAR T-cell therapiescovered include bb2121 and bb21217 (jointly developed by Celgene and bluebird bio), JCARH125 and MCARH171 (developed by Juno Therapeutics; a Celgene company) and LCAR-B38M (jointly developed by Legend Biotech and Janssen Biotech).1-5To date, bb21217 and MCARH171 are in Phase I clinical development, JCARH125 and LCAR-B38M are being evaluated in ongoing Phase I/II clinical trials, and bb2121 is at the most advanced development stage, with a pivotal Phase II study underway.6-10
bb2121
bb2121 is an anti-BCMA CAR T-cell therapy, containing a lentiviral CAR construct with a murine scFv, 4-1BB co-stimulatory motif, and a CD3z T-cell activation domain that is currently in early phase clinical development for RRMM.11In the dose-escalation (DE) cohort of a first-in-man, Phase I study (NCT02658929; N=21), patients with RRMM underwent a leukapheresis procedure to harvest autologous effector T-cells for the manufacturing of bb2121, and received a lymphodepleting conditioning regimen of fludarabine and cyclophosphamide prior to a single infusion of bb2121 at four dose levels (50 x106, 150 x106, 450 x106, and 800 x106CAR T-cells).11,12After 35 weeks follow-up, 18 patients treated at doses ≥150 x 106CAR T-cells were evaluable for efficacy and safety.11,12The overall response rate (ORR) was 94%, with 56% demonstrating a complete response (CR) or unconfirmed CR. The median progression free survival (PFS) was 11.8 months, with a median duration of response (MDR) of 10.8 months.11,12Across all DE cohorts, bb2121 displayed a manageable safety profile. As a consequence of these Phase I data, bb2121 was granted breakthrough therapy designation by the US Food and Drug Administration (FDA) and priority medicines eligibility by the European Medicines Agency (EMA) in 2017.13FDA approval of bb2121 is anticipated in the second half of 2020 for the treatment of RRMM.14,15Currently, a pivotal multi-center, Phase II study (KarMMa; NCT03361748) evaluating the efficacy and safety of bb2121 in patients with RRMM is underway, for which patient recruitment was completed in November 2018.14Moreover, participants are presently being recruited for an additional Phase II trial (KarMMa-2; NCT03601078) that will include a cohort of patients with high-risk multiple myeloma, and a Phase III trial (KarMMa-3; NCT03651128) that will evaluate the efficacy and safety of bb2121 versus standard triplet regimens in patients with RRMM.16,17
bb21217
The bb21217 anti-BCMA CAR T-cell therapy uses the same bb2121 CAR molecule, but has a different cell culture process, as bb21217-transduced T-cells are cultured ex vivowith a phosphoinositide 3-kinase inhibitor to enrich for T-cells displaying a memory-like phenotype (CD27and CD62LT-cells).18,19CAR T-cells enriched for CD27 and CD62Lphenotypes may be more persistent and potent than unselected CAR T-cells.18,20Preliminary results of the ongoing Phase I DE trial (NCT03274219) comprising 50 patients with RRMM with a median follow-up of 26 weeks, show that the first tested dose (150 x 106 CAR T-cells), resulted in an 83% ORR, with 90% of responses on-going, including 30% with a CR or stringent CR (sCR).4,18The adverse-event profile observed to date for bb21217 is consistent with known toxicities of CAR T-cell therapies.18
JCARH125
JCARH125 is a BCMA-targeting CAR T-cell therapy that has demonstrated a lack of inhibition by soluble BCMA and has an optimised manufacturing process that enriches for central T-cells displaying a memory-like phenotype.21In the ongoing Phase I/II trial (NCT03430011), 44 patients with RRMM were infused with JCARH125 in three DE cohorts. Median follow-up at first data release was only 11 weeks, yet the ORR was high at 82%.2At the lowest dose (50 x 106CAR T-cells), the ORR was 79%, with 43% of patients achieving sCR or CR.2JCARH125 has a little more toxicity than the other anti-BCMA CAR T cells (cytokine release syndrome [CRS] Grade ≥3 in 9% of patients; neurological events [NE] Grade ≥3 in 7% of patients).2,22
MCARH171
MCARH171 is an investigational BCMA-targeting CAR T-cell therapy that includes a truncated epidermal growth factor receptor safety system.5,23,24In a Phase I DE trial (NCT03070327), 11 patients with RRMM were treated with MCARH171 across four dose levels (mean doses: 72 x 106,137 x 106, 475 x 106, and 818 x 106CAR T-cells).5The ORR was 64%, and the MDR was 106 days.5As the efficacy results for MCARH171 were not as promising as those observed for JCARH125, and since both therapies are being studied by the same institution, further research is unlikely to focus on MCARH171.25
LCAR−B38M
LCAR-B38Mis a bispecific investigational CAR T-cell therapy directed against two distinct BCMA epitopes in patients with RRMM.26The bi-epitope BCMA binding moieties confer high avidity bindingand distinguish LCAR-B38M from the aforementioned BMCA CAR T-cell constructs.26,27In the ongoing Phase I/II LEGEND-2 trial (NCT03090659), 57 patients with RRMM were infused with LCAR-B38M at a median dose of 32.3 x 106CAR T-cells, with a median follow-up duration of 8 months.26The ORR was high at 88%, with CR achieved by 68%, VGPR achieved by 5%, and PR achieved by 14%.26The MDR was 14 months and median PFS for all treated patients was 15 months.26Moreover, LCAR-B38M CAR T-cell therapy demonstrated a manageable safety profile.26
The median PFS observed in this study was greater than that observed for patients in the Phase I study of bb2121 (15 months vs 11.8 months).12,26However, there was a key difference in the study population for the two therapies. In the LCAR-B38M study, the population was predominately Chinese, at an earlier stage of MM and wereless heavily pre-treated due to fewer anti-myeloma treatments being available in China.22Based on the initial results of the Phase I/II LEGEND-2 trial, the EMA granted a priority medicines designation for LCAR-B38M in April 2019.28
Conclusion
In Phase I clinical trials, CAR T-cell therapy has demonstrated encouraging clinical efficacy responses allied to a manageable safety profile for the treatment of patients with RRMM. Phase II/III trials will further determine the suitability of CAR T-cell therapy as a potential new treatment paradigm for MM.
References
1. bluebird bio. bluebird bio and Celgene Corporation Enter into Agreement to Co-Develop and Co-Promote Anti-BCMA CAR T Cell Therapy bb2121 in the United States. http://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-and-celgene-corporation-enter-agreement-co-develop. Accessed 18 April, 2019.
2. Celgene. Celgene Corporation Announces Initial Clinical Data from Ongoing Phase 1/2 Evolve Trial with Anti-BCMA CAR T Therapy JCARH125 in Relapsed/Refractory Multiple Myeloma at ASH 2018. https://ir.celgene.com/press-releases/press-release-details/2018/Celgene-Corporation-Announces-Initial-Clinical-Data-from-Ongoing-Phase-12-Evolve-Trial-with-Anti-BCMA-CAR-T-Therapy-JCARH125-in-RelapsedRefractory-Multiple-Myeloma-at-ASH-2018/default.aspx. Accessed 22 February, 2019.
3. Janssen. Janssen Enters Worldwide Collaboration and License Agreement with Chinese Company Legend Biotech to Develop Investigational CAR-T Anti-Cancer Therapy. https://www.janssen.com/janssen-enters-worldwide-collaboration-and-license-agreement-chinese-company-legend-biotech-develop. Accessed 18 April, 2019.
4. bluebird bio. bluebird bio and Celgene Corporation Present Initial Data from Ongoing Phase 1 Clinical Study of Next-Generation Anti-BCMA CAR T Cell Therapy bb21217 in Patients with Relapsed/Refractory Multiple Myeloma at ASH Annual Meeting. http://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-and-celgene-corporation-present-initial-data. Accessed 21 February, 2019.
5. Mailankody S, Ghosh A, Staehr M, et al. Clinical Responses and Pharmacokinetics of MCARH171, a Human-Derived Bcma Targeted CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma: Final Results of a Phase I Clinical Trial. Blood. 2018;132:959.
6. ClinicalTrials.gov. Study of bb21217 in Multiple Myeloma. https://clinicaltrials.gov/ct2/show/NCT03274219. Accessed 18 April, 2019.
7. ClinicalTrials.gov. BCMA Targeted CAR T Cells With or Without Lenalidomide for the Treatment of Multiple Myeloma. https://clinicaltrials.gov/ct2/show/NCT03070327. Accessed 18 April, 2019.
8. ClinicalTrials.gov. Study Evaluating the Safety and Efficacy of JCARH125 in Subjects With Relapsed and/or Refractory Multiple Myeloma (EVOLVE). https://clinicaltrials.gov/ct2/show/NCT03430011. Accessed 18 April, 2019.
9. ClinicalTrials.gov. Efficacy and Safety Study of bb2121 in Subjects With Relapsed and Refractory Multiple Myeloma (KarMMa) (bb2121). https://clinicaltrials.gov/ct2/show/NCT03361748. Accessed April 18, 2019.
10. ClinicalTrials.gov. LCAR-B38M-02 Cells in Treating Relapsed/Refractory (R/R) Multiple Myeloma (LEGEND-2). https://clinicaltrials.gov/ct2/show/NCT03090659. Accessed 18 April, 2019.
11. Raje NS, Berdeja JG, Lin Y, et al. bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: Updated results from a multicenter phase I study. J Clin Oncol. 2018;36:8007.
12. Celgene. Updated Results of Ongoing Multicenter Phase I Study of bb2121 anti-BCMA CAR T Cell Therapy Continue to Demonstrate Deep and Durable Responses in Patients with Late-Stage Relapsed/Refractory Multiple Myeloma at ASCO Annual Meeting. https://ir.celgene.com/press-releases/press-release-details/2018/Updated-Results-of-Ongoing-Multicenter-Phase-I-Study-of-bb2121-anti-BCMA-CAR-T-Cell-Therapy-Continue-to-Demonstrate-Deep-and-Durable-Responses-in-Patients-with-Late-Stage-RelapsedRefractory-Multiple-Myeloma-at-ASCO-Annual-Meeting/default.aspx. Accessed 21 February, 2019.
13. Celgene. Celgene Corporation and bluebird bio Announce bb2121 Anti-BCMA CAR-T Cell Therapy Has Been Granted Breakthrough Therapy Designation from FDA and Prime Eligibility from EMA for Relapsed and Refractory Multiple Myeloma. https://ir.celgene.com/press-releases/press-release-details/2017/Celgene-Corporation-and-bluebird-bio-Announce-bb2121-Anti-BCMA-CAR-T-Cell-Therapy-Has-Been-Granted-Breakthrough-Therapy-Designation-from-FDA-and-Prime-Eligibility-from-EMA-for-Relapsed-and-Refractory-Multiple-Myeloma/default.aspx. Accessed 21 February, 2019.
14. Celgene. Celgene Corporation and bluebird bio Complete Enrollment of Pivotal KarMMa Study of anti-BCMA Car T Cell Therapy bb2121 in Patients with Relapsed and Refractory Multiple Myeloma. https://ir.celgene.com/press-releases/press-release-details/2018/Celgene-Corporation-and-bluebird-bio-Complete-Enrollment-of-Pivotal-KarMMa-Study-of-anti-BCMA-Car-T-Cell-Therapy-bb2121-in-Patients-with-Relapsed-and-Refractory-Multiple-Myeloma/default.aspx. Accessed 17 April, 2019.
15. Celgene. Celgene Corporation Q4 2018 Earnings Conference Call. https://ir.celgene.com/events-and-presentations/event-details/2019/Celgene-Corporation-Q4-2018-Earnings-Conference-Call/default.aspx. Accessed 18 April, 2019.
16. ClinicalTrials.gov. An Efficacy and Safety Study of bb2121 in Subjects With Relapsed and Refractory Multiple Myeloma and in Subjects With High-Risk Multiple Myeloma (KarMMa-2). https://clinicaltrials.gov/ct2/show/NCT03601078. Accessed April 18, 2019.
17. ClinicalTrials.gov. Efficacy and Safety Study of bb2121 Versus Standard Triplet Regimens in Subjects With Relapsed and Refractory Multiple Myeloma (RRMM) (KarMMa-3). https://clinicaltrials.gov/ct2/show/NCT03651128. Accessed 18 April, 2019.
18. Shah N, Alsina M, Siegel DSD, et al. Initial Results from a Phase 1 Clinical Study of bb21217, a Next-Generation Anti Bcma CAR T Therapy. Blood. 2018;132:488.
19. Friedman KM, Garrett TE, Evans JW, et al. Effective Targeting of Multiple B-Cell Maturation Antigen-Expressing Hematological Malignances by Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum Gene Ther. 2018;29:585-601.
20. Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563-571.
21. Mailankody S, Htut M, Lee KP, et al. JCARH125, Anti-BCMA CAR T-cell Therapy for Relapsed/Refractory Multiple Myeloma: Initial Proof of Concept Results from a Phase 1/2 Multicenter Study (EVOLVE). Blood. 2018;132:957.
22. OncLive. Dr. Raje Discusses Investigational Treatment Strategies in Multiple Myeloma. https://www.onclive.com/inside-oncology/ash-2018-news/dr-raje-discusses-investigational-treatment-strategies-in-multiple-myeloma. Accessed 22 February, 2019.
23. Smith EL, Staehr M, Masakayan R, et al. Development and Evaluation of an Optimal Human Single-Chain Variable Fragment-Derived BCMA-Targeted CAR T Cell Vector. Mol Ther. 2018;26:1447-1456.
24. Cancer Therapy Advisor. MCARH171, a BCMA-Targeted CAR-T in Relapsed/Refractory Multiple Myeloma: Phase 1 Readout. https://www.cancertherapyadvisor.com/ash-2018/mcarh171-bcma-multiple-myeloma-cart-treatment-phase-1-risk/article/819080/. Accessed 22 February 2018.
25. Helwick C. Multiple Myeloma Pipeline Filled With CAR T-Cell Therapies. www.ascopost.com/issues/february-10-2019/multiple-myeloma-pipeline-filled-with-car-t-cell-therapies/. Accessed 22 February, 2019.
26. Zhao WH, Liu J, Wang BY, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11:141.
27. Zhao WH, Liu J, Wang BY, et al. Updated Analysis of a Phase 1, Open-Label Study of LCAR-B38M, a Chimeric Antigen Receptor T Cell Therapy Directed Against B-Cell Maturation Antigen, in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2018;132:955.
28. Johnson & Johnson. Janssen Announces Investigational CAR-T Therapy JNJ-68284528 Granted PRIME Designation by the European Medicines Agency. https://www.jnj.com/janssen-announces-investigational-car-t-therapy-jnj-68284528-granted-prime-designation-by-the-european-medicines-agency. Accessed 18 April, 2019.