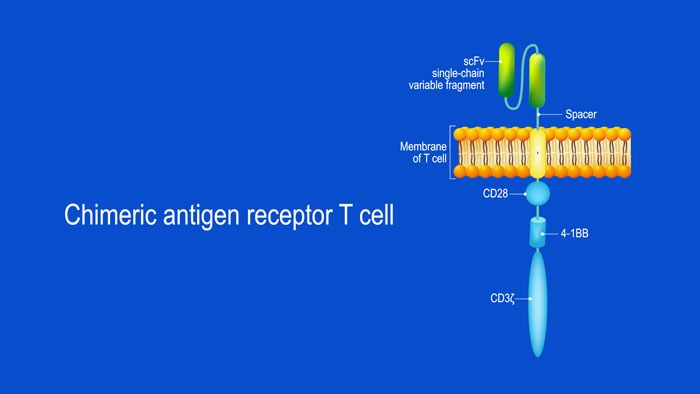
CAR T-cell structure
CARs are typically composed of four regions: (1) an extracellular antigen-binding domain; (2) a hinge or spacer peptide; (3) a transmembrane domain that anchors the CAR to the cell membrane; and (4) one or more intracellular signalling domains that leads to T-cell activation.5-7The extracellular antigen-binding domain is derived from a single-chain variable fragment (scFv) constructed through fusion of the antigen-binding regions of both heavy (VH) and light (VL) chains of a monoclonal antibody.6,8The intracellular signalling domain is most commonly derived from lymphocyte signal-initiating molecules including the CD3 zeta-chain (CD3z) of the T-cell receptor (TCR)/CD3 complex.9Incorporation of additional co-stimulatory signalling proteins, such as the CD28 family or the tumour necrosis factor receptor (TNFR) family of genes, can augment the ability of CARs to stimulate cytokine secretion, potentiate T-cell persistence and enhance anti-tumour efficacy.7,10
In chimeric antigen receptor (CAR) T-cell immunotherapy, autologous effector T-cells are harvested from the peripheral blood of patients with cancer through leukapheresis and genetically engineered ex vivousing a lentiviral or gammaretroviral vector to express synthetic proteins known as CAR receptors.1-4These receptors are directed against pre-determined cell-surface antigens expressed on tumor cells.1-4Lymphodepleting chemotherapy is given to the patient and then the re-programmed CAR T-cells are infused back into the patient, where they induce a selective immune response against tumor cells expressing the corresponding antigen.3,4
Approved CAR T-cell therapies
CAR T-cell immunotherapy has shown promising results in clinical trials with high rates of durable responses achieved in patients with pre-treated and chemotherapy-resistant chronic and acute B-cell precursor lymphoblastic leukaemia (B-ALL) and B-cell lymphoma.11-15Consequently, in August 2017, the FDA approved tisagenlecleucel, a CD19-directed CAR T-cell therapy for the treatment of relapsed or refractory B-cell ALL in patients under 25 years old.16Shortly thereafter, in October 2017, axicabtagene ciloleucel, another CD-19-directed T-cell therapy was approved for use in adult patients with relapsed or refractory large B-cell lymphoma.17More recently, in May 2018, tisagenlecleucel gained additional approval for the treatment of relapsed or refractory large B-cell lymphoma in adults.18
Key target of CAR T-cell therapy in multiple myeloma
Currently, CAR T-cell therapy is being investigated for the treatment of relapsed or refractory multiple myeloma (MM).14,19-22The principal target of CAR T-cell therapies for multiple myeloma is the B-cell maturation antigen (BCMA).22-24This is a transmembrane glycoprotein expressed exclusively by B-lineage-derived cells, particularly in the interfollicular region of the germinal centers, and on plasmablasts and differentiated plasma cells (PCs).24-27BCMA is selectively induced during PC differentiation, supporting humoral immunity by mediating survival of normal bone marrow PCs and plasmoblasts.23-25,28As BCMA expression is virtually absent on naïve and most memory B-cells, it is not critical for overall B-cell homeostasis but is required for optimal survival of long-lived PCs.26-30In patients with MM, BCMA is widely expressed on malignant PCs at significantly higher levels.22-24Indeed, BCMA expression increases as MM progresses, from normal to monoclonal gammopathy of undetermined significance to smouldering to active MM.22Due to the lineage-restricted expression profile of BCMA and its survival function in plasma cells, it is a potentially viable target for CAR T-cell therapy in patients with MM.22-25Given the poor outcome of patients with relapsed or refractory multiple myeloma, there is a high unmet need for effective treatments. It is hoped that novel treatment options such as CAR T-cell immunotherapy may offer hope for heavily pre-treated patients.
References
1. Jin C, Fotaki G, Ramachandran M, et al. Safe engineering of CAR T cells for adoptive cell therapy of cancer using long-term episomal gene transfer. EMBO Mol Med. 2016;8:702-711.
2. Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Mol Ther Methods Clin Dev. 2017;4:92-101.
3. Buechner J, Kersten MJ, Fuchs M, Salmon F, Jäger U. Chimeric Antigen Receptor-T Cell Therapy. HemaSphere. 2018;2:e18.
4. Hay KA, Turtle CJ. Chimeric Antigen Receptor (CAR) T Cells: Lessons Learned from Targeting of CD19 in B-Cell Malignancies. Drugs. 2017;77:237-245.
5. Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11:855-873.
6. Gacerez AT, Arellano B, Sentman CL. How Chimeric Antigen Receptor Design Affects Adoptive T Cell Therapy. J Cell Physiol. 2016;231:2590-2598.
7. Guedan S, Calderon H, Posey AD, Jr., Maus MV. Engineering and Design of Chimeric Antigen Receptors. Mol Ther Methods Clin Dev. 2019;12:145-156.
8. Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107-126.
9. Dai H, Wang Y, Lu X, Han W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. J Natl Cancer Inst. 2016;108.
10. Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453-1464.
11. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725-733.
12. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507-1517.
13. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439-448.
14. Kriegsmann K, Kriegsmann M, Cremer M, et al. Cell-based immunotherapy approaches for multiple myeloma. Br J Cancer. 2019;120:38-44.
15. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380:45-56.
16. U.S. Food and Drug Administration. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm574154.htm. Accessed 20 February.
17. U.S. Food and Drug Administration. FDA approves axicabtagene ciloleucel for large B-cell lymphoma. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm581296.htm. Accessed 20 February.
18. U.S. Food and Drug Administration. FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm606540.htm. Accessed 20 February.
19. Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688-1700.
20. Fan F, Zhao W, Liu J, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed multiple myeloma. J Clin Oncol. 2017;35:LBA3001.
21. Raje NS, Berdeja JG, Lin Y, et al. bb2121 anti-BCMA CAR T-cell therapy in patients with relapsed/refractory multiple myeloma: Updated results from a multicenter phase I study. J Clin Oncol. 2018;36:8007.
22. Tai YT, Anderson KC. Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy. 2015;7:1187-1199.
23. Trudel S, Lendvai N, Popat R, et al. Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19:1641-1653.
24. Bu DX, Singh R, Choi EE, et al. Pre-clinical validation of B cell maturation antigen (BCMA) as a target for T cell immunotherapy of multiple myeloma. Oncotarget. 2018;9:25764-25780.
25. Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048-2060.
26. Ryan MC, Hering M, Peckham D, et al. Antibody targeting of B-cell maturation antigen on malignant plasma cells. Mol Cancer Ther. 2007;6:3009-3018.
27. Chiu A, Xu W, He B, et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood. 2007;109:729-739.
28. Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263-275.
29. O’Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91-98.
30. Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286-297.