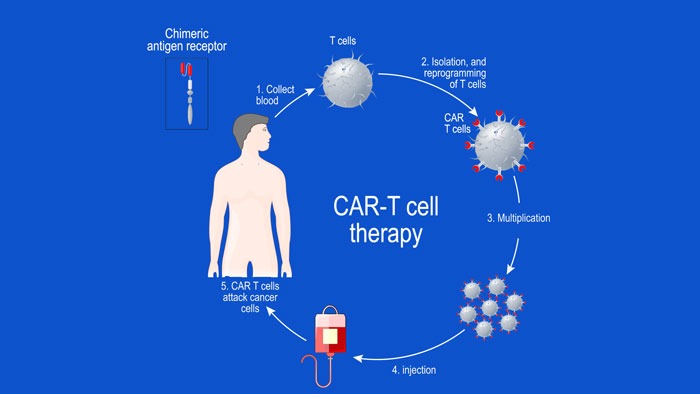
by Atif Riaz | Sep 30, 2020 | Featured, Featured News
In this article, we look at some of the early-phase clinical trials, where chimeric antigen receptor (CAR) T-cell therapy has been targeted to the B-cell maturation antigen (BCMA) in patients with relapsed or refractory MM (RRMM). The anti-BCMA CAR T-cell...

by MM360 Staff Writer | Sep 28, 2020 | Featured News, Myeloma News
ONCOTracker and The Binding Site have entered an agreement to develop and commercialize a new test that measures serum levels of the B-cell maturation antigen (sBCMA) protein to monitor people with blood cancers, including multiple myeloma. Serum B-cell maturation...

by MM360 Staff | Sep 27, 2020 | Featured News, Myeloma News
Updated results from a previously published cohort was published in Clinical Transplant. 92 newly diagnosed myeloma patients who received tandem transplant were compared them with 81 contemporary patients who received autologous transplant only. With a median...

by MM360 Staff | Sep 22, 2020 | Featured News, Myeloma News
FDA set a target action date of March 27, 2021 Ide-cel is the first CAR T cell therapy accepted for regulatory review for multiple myeloma Bristol Myers Squibb and bluebird bio, Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted for Priority...

by MM360 Staff Writer | Sep 22, 2020 | Featured News, Myeloma News
FDA set a target action date of March 27, 2021 Ide-cel is the first CAR T cell therapy accepted for regulatory review for multiple myeloma Bristol Myers Squibb and bluebird bio, Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted for Priority...
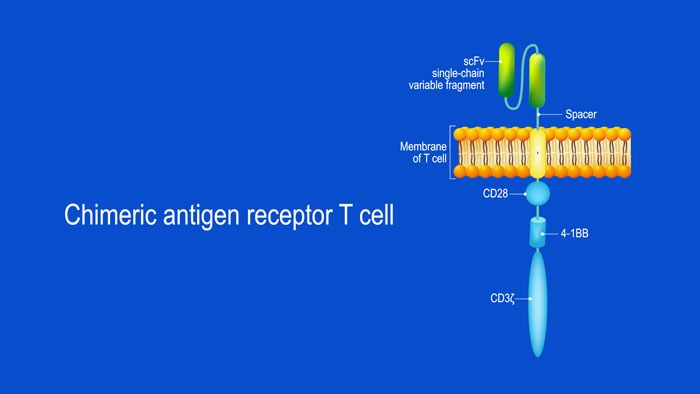
by Atif Riaz | Sep 21, 2020 | Featured, Featured News
CAR T-cell structure CARs are typically composed of four regions: (1) an extracellular antigen-binding domain; (2) a hinge or spacer peptide; (3) a transmembrane domain that anchors the CAR to the cell membrane; and (4) one or more intracellular signalling domains...






