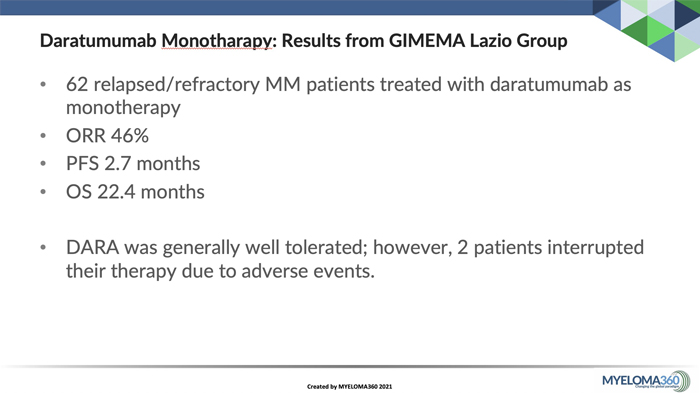
A recent article published Feb. 2, 2021 in the Annals of Hematology demonstrated efficacy and safety of daratumumab (DARA) monotherapy in real-world patients. The GIMEMA Lazio group reported experience in 62 patients with relapsed/ refractory myeloma who had previously received at least two treatment lines including a PI and an IMiDs or had been double refractory.
Patients received DARA 16 mg/kg intravenously weekly for 8 weeks, every 2 weeks for 16 weeks, and every 4 weeks until disease progression or unacceptable toxicity.
Results
ORR 46%
PFS 2.7 months
OS 22.4 months
DARA was generally well tolerated; however, 2 patients interrupted their therapy due to adverse events.
Conclusion
DARA monotherapy is an effective strategy for heavily pre-treated and refractory patients with multiple myeloma, with a favorable safety profile.
Reference: Vozella, F., Siniscalchi, A., Rizzo, M. et al. Daratumumab in multiple myeloma: experience of the multiple myeloma GIMEMA Lazio group. Ann Hematol (2021). https://doi.org/10.1007/s00277-020-04374-y