A once-weekly regimen of selinexor, bortezomib, and dexamethasone slows the progression of relapsed or refractory multiple myeloma, according to a study published in the Nov. 14 issue of The Lancet.
The results from the BOSTON study demonstrate that the once-weekly regimen of selinexor, bortezomib, and dexamethasone (SVd) reduced the risk of disease progression or death by 30% and induced a higher rate of overall and deep responses compared to patients receiving a standard twice-weekly bortezomib and low-dose dexamethasone regimen (Vd). This was observed despite approximately 40% less bortezomib, 25% less dexamethasone and approximately 35% fewer clinic visits on the SVd arm as compared with the standard Vd therapy arm.
“Encouragingly, the efficacy of the SVd regimen was consistent and noteworthy across several key subgroups, including patients who were frail or 65 years and older, patients with high-risk cytogenetics, patients with moderate renal impairment and patients who had either prior bortezomib or lenalidomide treatment,” said Dr. Paul Richardson, MD, Clinical Program Leader and Director of Clinical Research, Jerome Lipper Multiple Myeloma Center at the Dana-Farber Cancer Institute and co-senior author of the manuscript.
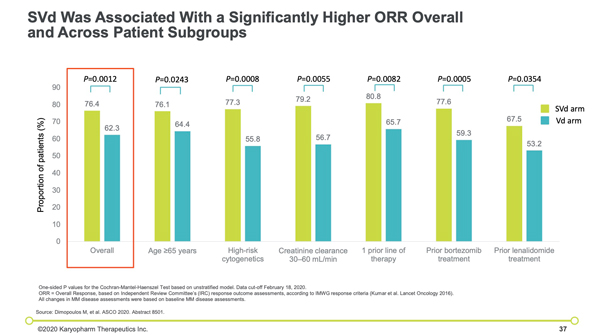
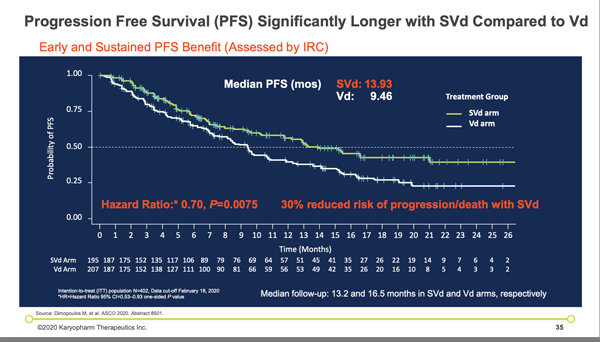
“Despite the enrollment of 50% of patients with high risk cytogenetics, a particularly difficult to treat population, the SVd regimen demonstrated a 47% improvement in progression-free survival as compared to the Vd regimen and an overall response rate of 76.4%. The researchers found that the median progression-free survival was 13.93 and 9.46 months in the selinexor, bortezomib, and dexamethasone group and the bortezomib and dexamethasone group, respectively (hazard ratio, 0.70).
Additionally, the rate and severity of peripheral neuropathy, a key treatment-limiting side effect commonly seen with bortezomib therapy, was significantly lower on the SVd arm compared to the Vd arm and may lead to improved patient quality of life,” said Sundar Jagannath, MD, Director of the Center of Excellence for Multiple Myeloma and Professor of Medicine, Hematology and Medical Oncology, at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, and an investigator in the BOSTON study.
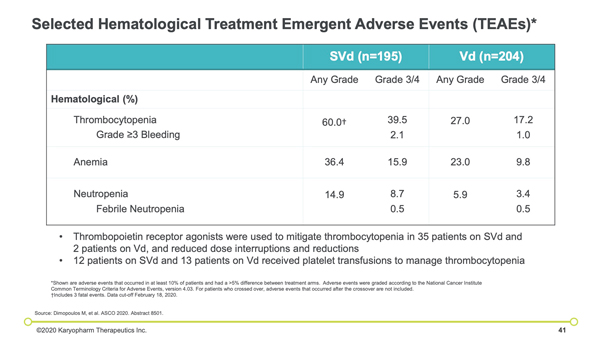
Thrombocytopenia (39 versus 17 percent), fatigue (13 versus 1 percent), anemia (16 versus 10 percent), and pneumonia (11 versus 11 percent) were the most frequent grade 3 to 4 adverse events reported in the treatment groups. Peripheral neuropathy of grade 2 or greater occurred less often among patients treated with selinexor, bortezomib, and dexamethasone (21 versus 34 percent; odds ratio, 0.50).
The authors concluded that “a once-per-week regimen of selinexor, bortezomib, and dexamethasone is a novel, effective, and convenient treatment option for patients with multiple myeloma who have received one to three previous lines of therapy”.
Reference:
Grosicki, S et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): a randomised, open-label, phase 3 trial. Lancet 2020;396:1563-1573. DOI:https://doi.org/10.1016/S0140-6736(20)32292-3
